June is HHT Awareness Month
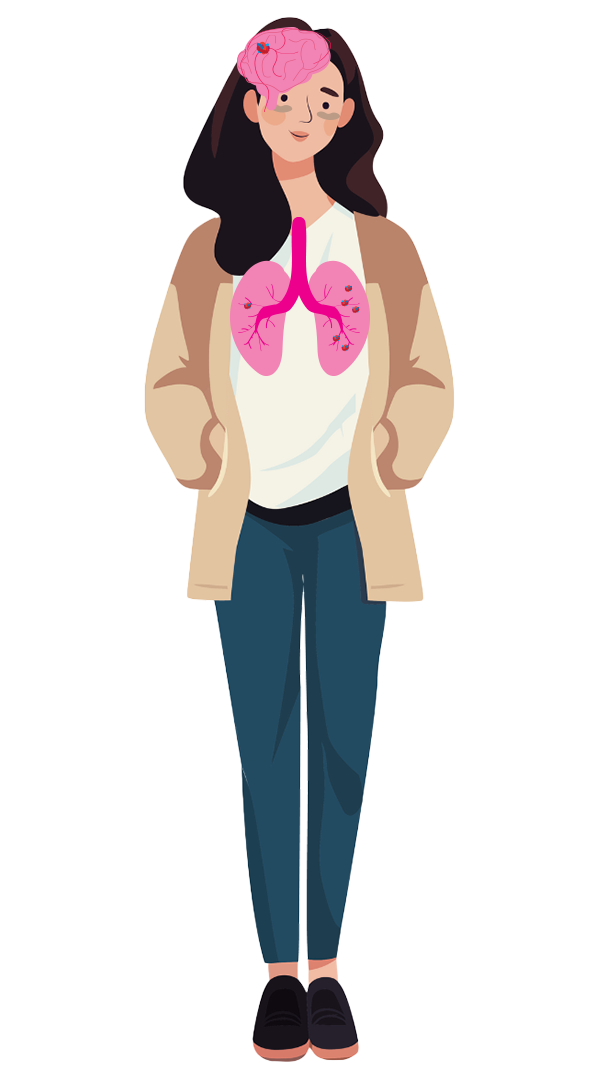
For individuals and families affected by Hereditary Hemorrhagic Telangiectasia (HHT), awareness and support are vital. June is our opportunity to come together, share our experiences, and educate others about this often-underdiagnosed condition. Explore resources, connect with the community, and find out how you can help amplify the voices of those living with HHT.
What Can You Do To Get Involved?
Every year on June 23 — and throughout the month of June — local, state, federal, and national organizations come together to shed light on the impact of HHT and to show support for those at living with and caring for those with HHT.
This year’s theme, “Beyond the Visible,” focuses on shining a light on those living with the unseen challenges that come with HHT. As we observe the our annual awareness campaign, this theme reminds us of how far we’ve come and the work still ahead to diagnose, treat, and improve the quality of life for HHT patients. By working together, we can create a better future.

Raise Awareness

Host a Fundraiser

Donate
Upcoming Events
Kate is a teacher, an avid swimmer, and addicted to green tea lattes. She loves traveling, dogs, and dreams of having a family.
Kate lives with HHT.