UPDATES
Topic Areas
Update: November 18, 2021 | COVID-19 VACCINATIONS & CHILDREN
Andrew White, M.D. – Co-Director, HHT Center of Excellence, Washington University School of Medicine
The FDA recently approved the Pfizer-BioNTech COVID-19 Vaccine for emergency use in children 5 - 11 years of age at a lower dose. Adolescents ages 12 years+ have previously been approved to receive the same dosage of Pfizer-BioNTech COVID-19 vaccine as adults.
We have reviewed and agree with the American Academy of Pediatrics’ recommendations related to the coronavirus disease 2019 (COVID-19) vaccine in children and adolescents. You can review the complete statement here.
Update: May 13, 2021 | COVID-19 VACCINATIONS & BLEEDING
The concern about the vaccine is that the deeper injection could lead to bleeding at the injection site in people who have problems of blood coagulation, meaning people who have disorders where their blood does not clot normally, and they could potentially bleed significantly where the injection is given.
Because people with HHT do not have problems with coagulation of their blood, the injection is safe for the typical person with HHT. If you have HHT and are on a medication to alter your coagulation (such as coumadin for atrial fibrillation), you should check with your doctor. The warning should have been given for people with coagulation problems. Most likely the term bleeding was used because this is the more common lay term and is often considered to be synonymous with the word “bleeding” even though they are indeed different. The fact that the word bleeding was used which has led to the confusion regarding HHT is indicative of the work that needs to be done to raise awareness of HHT, even among medical professionals.
Update: April 22, 2021 | COVID-19 VACCINATIONS & BLOOD CLOTS
The FDA recently paused use of the Johnson & Johnson COVID-19 vaccine due to reports of rare blood clots observed in 6 patients out of nearly 7 million Americans given this vaccine. Internationally, the AstraZeneca vaccine was also paused due to reports of blood clotting resulting in a risk of 4 in 1 million receiving the vaccine. There have been no recognized associations between the Pfizer or Moderna COVID-19 vaccine and blood clotting.
Given our current knowledge about why the clots may be occurring, there is no reason to believe that patients with HHT who have received any of the following vaccines--Johnson & Johnson, AstraZeneca, Pfizer or Moderna--would be at any increased risk of this complication. The overall safety and efficacy profile of the COVID-19 vaccines approved in the United States is excellent, and COVID-19 is an extremely contagious and potentially deadly disease.
In summary, the risk of developing blood clots WITH COVID vaccines appears to be very rare while the risk of developing blood clots from COVID-19 infection is much higher (ranging between 2-30% in patients hospitalized with the disease). Therefore, for nearly all patients, the benefit of vaccination far outweighs any risk of severe vaccine-associated complications, including blood clots.
Update: February 11, 2021 | HHT PATIENTS AND COVID VACCINES
Marie Faughnan, MD, MSc – Director, Toronto HHT Centre of Excellence Director
Pfizer and Moderna Vaccines
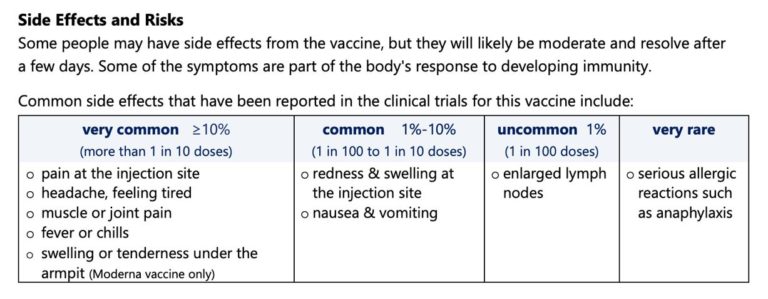
Chart/Image from Toronto Public Health InfoSheet 2021
Addressing Common Myths About the COVID Vaccine
- Useless as its effect will wear off quickly: Maybe, revaccinate.
- The COVID vaccine will alter my DNA: No.
- The vaccine will cause COVID infection: No (no live virus)
- Virus mutants will make the vaccine useless: Re-engineer if needed.
- If I have reacted to flu vaccine, then I will react to COVID vaccine: Not likely, different ingredients.
- There is no point in getting the COVID vaccine if I have had COVID: No, can extend /boost immunity.
- People with a bleeding disorder (aka HHT?) can’t get COVID vaccine: No, the issue is bruising at injection site for people with clotting disorder/medication. This concern does not apply to HHT.
HHT Patients and COVID Vaccines
There is no HHT-related reason to avoid the COVID vaccination. The COVID vaccination should be as safe and as effective for people with HHT as the general population. Some people with HHT may be at higher risk of getting really sick or dying from COVID, so the benefits should outweigh the risks.
Please review the recent guidance from the North American Cure HHT Scientific and Medical Advisory Council regarding COVID-19 vaccinations for additional information (update of 12/16/2020).
Update: December 16, 2020 | COVID-19 VACCINATION
The COVID-19 Vaccine is a major development in the fight against the pandemic. This statement has been developed by the Scientific and Medical Advisory Committee of Cure HHT to answer some of the questions that patients may have.
While the COVID-19 vaccine was not specifically tested in HHT patients, it was tested in patients with chronic diseases. There are no data to suggest that the vaccine will behave differently in HHT than it does in other patient populations. The committee feels that the vaccine should be offered to HHT patients according to CDC guidelines. Possible contraindication for vaccination should be the same as for the general public.
The CDC has recommended that patients with significant underlying illnesses receive the vaccine earlier during initial distribution. While HHT is a chronic disease, the committee feels that the diagnosis of HHT by itself would not place a patient at an increased risk for severe illness. However, there are some patients with HHT and associated co-morbidities that should be considered higher risk and therefore placed into the pool of people to be vaccinated earlier. These would include:
- Patients with heart failure
- Patients with Pulmonary Arterial Hypertension
- Patients with Pulmonary AVMs who experience chronic low blood oxygen levels (Pulse oximetry < 90%)
- Patients with HHT who frequently access the health care system which may include those receiving frequent iron infusions, blood transfusions or bevacizumab treatments.
The committee recommends that patients discuss with their physician any concerns they may have regarding vaccination and timing of obtaining the vaccine.
Update: November 24, 2020 | COVID-19 RARE DISEASE RESEARCH
Rare disease patients and caregivers: How are you being impacted by the novel coronavirus pandemic? Please complete a 20-minute online research survey from home to share your experiences. This study is being conducted by National Institutes of Health (NIH)’s Rare Diseases Clinical Research Network (RDCRN). Your responses may help researchers understand the impacts of COVID-19 on the rare disease community. Survey closes Dec 15, 2020. Complete the survey or learn more at https://RareDiseasesNetwork.org/COVIDsurvey
Update: August 6, 2020 | COVID-19 TESTING
Nasal swab testing for COVID-19 can be problematic for HHT patients because of the risk of inducing a nosebleed, and many members of the HHT community have asked about the availability of oral swabs. Some have been disappointed to learn that they may not be as easily available as hoped. This can occur because of the lab setup approval process at each individual lab or collection location.
Testing for COVID-19
There are two basic types of testing for COVID-19:
- A viral test tells you if you have a current infection.
- An antibody test might tell you if you had a past infection.
Testing for Current Infection (viral testing)
Testing for current infection can use two different techniques, including a molecular (or PCR) test or an Antigen test (rapid test). Both testing methods use a nasal and/or throat swab to get sample fluid, and the molecular test can collect fluid from saliva but this is done less often.
Molecular tests are considered very accurate when properly performed by a health care professional, but the rapid Antigen test appears to miss some infections. A positive Antigen test result is considered very accurate, but there’s an increased chance of false-negative results.
Obtaining Fluid Samples (viral testing)
Samples for molecular or antigen testing (looking for current infections) can be obtained from the nose, the mouth or from saliva. Saliva testing may be the least sensitive as it may not contain high enough amounts of the virus to allow for detection.
Most labs are approved for the nasal swab testing. Though oral swabs are available in some locations, when oral swabs are performed, the processing lab must have the proper test and equipment sensitive enough to detect the virus from the oral swabs, or it may miss some cases and/or the results may not be valid. Not all labs are equipped to process oral swabs. If an oral swab is imperative, it may mean having to look to other lab locations that are able to process oral swabs.
Unfortunately, for many institutions, adding a second testing method (oral swab) would require a significant infrastructure and staff learning investment. The HHT Centers of Excellence are not able to add this capacity on their own. Hopefully, more centers will be able to offer this testing option in the future for individuals who are unable to undergo nasal swab testing.
Testing for Previous Infection (antibody testing)
Testing to look for previous infections are based on antibody testing. Antibody tests may provide quick results, but should not be used to diagnose an active infection. Antibody tests only detect antibodies the immune system develops in response to the virus, not the virus itself. It can take days to several weeks to develop enough antibodies to be detected in a test. Therefore, antibody testing, also known as serology testing, is typically done after full recovery from COVID-19.
If test results show that you have antibodies, it indicates that you were likely infected with COVID-19 at some time in the past. It may also mean that you have some immunity. But the World Health Organization cautions that there’s a lack of evidence on whether having antibodies means you’re protected against reinfection with COVID-19. The level of immunity and how long immunity lasts are not yet known. Ongoing studies will eventually reveal more data on this.
The timing and type of antibody test affects accuracy. If you have testing too early in the course of infection, when the immune response is still building up in your body, the test may not detect antibodies, so you may have to wait several days to get tested.
Obtaining Fluid Samples – Antibody Testing
A blood sample is taken and then tested to determine whether you have developed antibodies against the virus. The immune system produces these antibodies — proteins that are critical for fighting and clearing out the virus.
Update: June 11, 2020 (chart updated 7/20/200) | NORD ASSISTANCE PROGRAM & CENTERS OF EXCELLENCE (OPERATING STATUS)
NORD ASSISTANCE PROGRAM
For patients who have been directly impacted by the COVID-19 pandemic, the National Organization for Rare Disorders (NORD) is offering an Assistance Program designed to provide assistance to rare disease patients. “Direct impact” includes job loss, reduced work hours, quarantine, etc. The program is unable to assist with financial needs that are not a result of direct impact from the COVID-19 pandemic. There are two programs available, “Critical Needs” and “Premium & Limited Medical Assistance,” however, assistance may only be provided in one of these programs. See the program descriptions below:
Program #1: The COVID-19 Rare Disease Premium & Limited Medical Assistance Fund
Provides financial assistance for certain out-of-pocket costs associated with patient’s health insurance premiums.
Provides eligible uninsured and under-insured patients (for whom insurance has declined coverage for this expense) with financial support for out-of-pocket limited medical expenses such as:
- Medical visits & telehealth consultations
- Laboratory & diagnostic testing
- Physical and/or occupational therapy and/or other physician prescribed therapy
- Durable medical equipment
- Medical supplies (tube feeding supplies, dressing kits, personal protective equipment)
Program #2: The COVID-19 Rare Disease Critical Needs Non-Medical Assistance Fund
Provides eligible individuals impacted by the COVID-19 pandemic, with financial assistance for unexpected or emergency and essential expenses such as:
- Unexpected utility expenses
- Communication expenses (e.g. phone, cell phone, internet)
- Emergency repairs to car, furnace, home or major appliances
- Assistance with travel and/or lodging logistics and expenses
- Rent or mortgage payment assistance
- Support for adaptive learning during school closures
All Program Eligibility Criteria (Mandatory):
- Patients must have a confirmed rare disease diagnosis.
- Individuals must be US citizens or provide documentation of permanent US residency status; or resident of the United States for at least six months.
- Applicants must be willing to provide proof of residency such as a utility bill indicating their name and address.
- Applicants must meet financial eligibility criteria (at or below 400% of the Federal Poverty Level guidelines (FPL) or demonstrate financial impact/mitigating circumstances due to the COVID-19 pandemic (examples include job loss, inability to work due to stay-at-home orders, school closures etc.).
Should you wish to be considered for eligibility, please contact the RareCare Patient Assistance Team / COVID-19 Assistance at (203) 242-0497 or via email at [email protected]. Please provide your full name, a phone number where you can be reached, the rare disease with which you/your family member has been diagnosed, and the Covid-19 impact with which you/your family have been affected (all information is required).
CENTERS OF EXCELLENCE (OPERATING STATUS)
All HHT Centers of Excellence (CoEs) have protocols in place to prevent the spread of infection in the centers, hospitals and emergency rooms. If patients have questions or concerns, they should call the CoE directly. In the event of an emergency, please seek immediate treatment and do not risk your safety out of fear of contracting the novel coronavirus. Click here to view the contact information for a CoE near you.
CENTERS OF EXCELLENCE (OPERATING STATUS)
All HHT Centers of Excellence (CoEs) have protocols in place to prevent the spread of infection in the centers, hospitals and emergency rooms. If patients have questions or concerns, they should call the CoE directly. In the event of an emergency, please seek immediate treatment and do not risk your safety out of fear of contracting the novel coronavirus. Click here to view the contact information for a CoE near you.
Update: April 17, 2020 | BLOOD CLOTS, COVID-19 AND THE HHT COMMUNITY
Dr. Raj Kasthuri – Director, University of North Carolina, Chapel Hill HHT Center of Excellence
Dr. Hanny Al-Samkari – Associate Director, Massachusetts General Hospital HHT Center of Excellence
Guidance from the North American Cure HHT Scientific and Medical Advisory Council
There is growing concern that individuals affected by COVID-19 are at increased risk for thrombotic (blood clot formation) complications and what is referred to as COVID coagulopathy. The incidence of this complication is reported to be as high as 27%.
This increased risk with COVID-19 infections is particularly relevant to patients with HHT who are receiving treatment with medications that are either associated with an increased risk for blood clot formation or interfere with the natural clot disintegration. These medications include: oral agents such as (1) thalidomide, (2) pomalidomide, (3) tamoxifen, (4) pazopanib (Votrient), (5) bevacizumab (Avastin) – intravenous delivery and other antiangiogenic medications, (6) aminocaproic acid (Amicar) and (7) tranexamic acid (Lysteda).
While there is no evidence that HHT confers an increased risk for acquiring the COVID-19 infection, we recommend certain precautions to minimize the risk for complications in patients with HHT who are diagnosed with the COVID-19 infection. They are as follows:
- HHT patients with suspected COVID-19 should alert their treating physician that they have HHT as their diagnosis may be important in any decision to provide prophylactic treatment to prevent clotting;
- HHT patients with suspected COVID-19 should undergo testing to confirm/refute the diagnosis as it has considerable management implications from an HHT standpoint;
- HHT patients with confirmed COVID-19 infection should notify their HHT center/treating physician of this situation promptly;
- HHT patients with confirmed COVID-19 infection should discuss with their prescribing HHT physician the possibility of temporarily discontinuing the following oral medications for 4 weeks: thalidomide, pomalidomide, tamoxifen, pazopanib, and other antiangiogenic medications as well as aminocaproic acid (Amicar), tranexamic acid (Lysteda);
- HHT patients with confirmed COVID-19 infection who are receiving treatment with intravenous bevacizumab (Avastin) should discuss with their prescribing HHT physician the possibility of delaying the next dose of bevacizumab (Avastin) by 4 weeks; and
- HHT patients who are hospitalized with COVID-19 are encouraged to share the contact information of their HHT center with the treating physicians.
Update: April 9, 2020 | GUIDELINES FOR COVID-19 TESTING: PATIENTS WITH HHT
Jeffrey Terrell, MD – Professor of Otolaryngology, Michigan Medicine
Guidelines for COVID-19 Testing in Relation to HHT Patients with Nosebleeds:
 HHT patients can have a nasal or nasopharyngeal swab administered if they:
HHT patients can have a nasal or nasopharyngeal swab administered if they:
- Do NOT get “gusher nosebleeds” (defined as brisk, high volume –more than a couple of tablespoons– bleeds)
- Do NOT get nosebleeds that require blood transfusion or iron infusions.
- Do NOT get frequent small nosebleeds that they have difficulty controlling on their own, with confidence . . .example: if a small nosebleed started, they would confidently manage it on their own, without medical professional help.
If HHT patients cannot meet the above criteria, it is suggested that they advise the administering healthcare provider that they have HHT, explain the nature and severity of their nosebleeds, and ask for an “oropharyngeal swab” (throat swab).
The nasal viral swab is thinner and more flexible than the typical cotton swab on a (relatively rigid) stick.
Patients that typically experience rare (or no) minor nosebleeds may experience a minor nosebleed after COVID-19 testing with little to no lasting issues.
View the CDC’s Guidelines for Collecting, Handling, and Testing Clinical Specimens here.
Update: April 2, 2020 | RISK FACTORS: HHT PATIENTS WHO CONTRACT COVID-19
Marie Faughnan, MD, MSc – Director, Toronto HHT Centre of Excellence Director
There is currently no research or evidence related to how people with hereditary hemorrhagic telangiectasia (HHT) experience COVID infection. In addition to the issues brought about by nosebleeds including increased touching of the face, Dr. Faughnan has compiled a list of conditions that may increase the risk of becoming very sick once an HHT patient becomes infected with COVID-19. Based on population reports and also knowledge of typical chronic disease factors that predispose to viral infections and pneumonia, the suspected risk factors for HHT patients are detailed below:
Having these conditions MAY increase the risk in people with HHT who have contracted COVID-19:
- Severe anemia (from chronic severe nose and/or gastrointestinal bleeding)
- Heart failure (from severe liver VMs)
- Chronic liver dysfunction (from severe liver VMs)
- Low oxygen levels (from untreated lung AVMs or diffuse lung AVMs)
- Seizure disorder which is not well controlled
Suggested Prevention: Controlling your disease and symptoms as well as possible with your doctor’s advice. The better controlled your chronic symptoms/condition are, the less the risk should be.
Word Key: AVM=arteriovenous malformation, VM=vascular malformation
*This list does not include information about non-HHT risk factors; people should review those with their physician and follow related advice. View the CDC list of general risk factors here.
Update: March 26, 2020 | HHT CENTERS OF EXCELLENCE
With many new and existing patients in the HHT Community inquiring about well visits, screenings, scheduled procedures, etc. at HHT Centers of Excellence (COEs) amid COVID-19, we have been in discussion with all twenty-eight HHT COEs to disseminate visit protocols associated with their institutions. With 90% of our COEs reporting in with their individual non-visit options (Virtual Health), we are compiling a comprehensive list to include details for each institution. This detailed list will be available on our website as soon as the information is available. In the meantime, if you are scheduled or need to schedule a visit to one of the HHT COEs, please check their individual websites for current visit information and scheduling of routine procedures. Check back for the comprehensive list with details for each COE.
Update: March 18, 2020 /2:36 PM (EDT) | HHT and COVID-19 RISK FACTORS: EPISTAXIS
Marcelo Serra, MD – Director, Unidad HHT Hospital Italiano de Buenos Aires
Epistaxis (Nosebleeds): Almost 95% of the patients with HHT present with nosebleeds although of different magnitudes between them. As they are spontaneous, the manipulation of the nose is a frequent event and often without previous hygienic precautions, due to the suddenness of the event or frequency of the situation. Manipulation of the airway (nose, mouth) without prior hygiene maybe the entry of the COVID-19. The surprising appearance of a nosebleed can lead to manipulation of the nose, face, etc. without first having been cleaned, and therefore, Epistaxis (nosebleeds) may represent a risk condition for entry or dissemination of the COVID-19. For this reason we recommend first to properly sanitize your hands despite the fact that it represents a drop of blood for a few more seconds and then to get the necessary instruments to vigorously compress the nose and clean yourself.
Always remain calm in these situations, be supportive and socially responsible.
Update: March 18, 2020 /10:26 AM (EDT) | HHT and COVID-19 RISK FACTORS: ANEMIA & IRON DEFICIENCY
Joint Statement – Hematology:
Raj Kasthuri MD – Director, University of North Carolina, Chapel Hill HHT Center of Excellence
Hanny Al-Samkari MD – Associate Director, Massachusetts General Hospital HHT Center of Excellence
The COVID-19 outbreak has been declared a pandemic by the WHO. Healthcare institutions across the country are preparing for the impact this will have on their patients, staff and resources in the coming weeks to months. Encounters that occur in the clinical setting confer the risk for exposure to individuals affected by the corona virus. All of this is likely to also affect the HHT community.
An HHT diagnosis in and of itself does not increase the risk of acquiring the Covid-19 infection, although other coexisting medical conditions may impact an individual’s risk independent of HHT.
Iron deficiency: anemia is not considered an immune compromised state. A number of people with HHT have iron deficiency anemia and receive frequent iron infusions. Some may also require periodic blood transfusions for severe anemia. We would like to share some thoughts and recommendations with this in mind:
- Blood transfusions for people with severe anemia should not be postponed as they can be life-saving interventions in the setting of recurrent/ongoing bleeding.
- All people with HHT who receive regular iron infusions should proactively communicate with their hematologist/oncologist (or other prescribing physician) to discuss the plan for monitoring of blood counts and for iron infusions scheduled for the next 1-2 months.
- Clinic, lab or infusion visits carry with them the risk of exposure to individuals affected by the virus and the risk/benefit of all visits should be discussed with the provider.
- Iron infusions in people with HHT are not an optional therapy but an essential component of their HHT care.
- Withholding iron infusions could result in adverse outcomes such as worsening anemia and related symptoms, need for blood transfusions, and/or hospitalizations. Therefore, iron infusions should not be delayed or otherwise postponed unless absolutely necessary.
Please continue to follow the CDC’s guidelines and recommendations on hygiene and how best to safeguard yourselves against the infection.
Update: March 12, 2020 | COVID-19 CDC RECOMMENDATIONS
We are regularly monitoring updates from our HHT Center of Excellence physicians, the Centers for Disease Control and Prevention (“CDC”), and the World Health Organization (“WHO”).
On Jan. 20, the World Health Organization declared the COVID-19 outbreak an international public health emergency. The U.S. Centers for Disease Control and Prevention (CDC) have issued Level 3 and Level 2 warnings to avoid or postpone nonessential travel for several countries in Asia, the Middle East and Europe only. No travel advisories currently exist for the United States or Canada.
With some spread of COVID-19 occurring in several communities in the United States, the CDC now recommends older adults and travelers with underlying health issues limit travel to avoid crowded places, which includes all cruise ship travel and non-essential travel such as long plane trips. No travel advisories are currently in place for any region of the U.S., but some employers, including many of our HHT Centers of Excellence, in response to current conditions and especially in areas affected by community spread, have begun to issue their own travel guidance or restrictions.
The CDC posted recommendations for people at risk for serious illness from COVID-19, including those over the age of 60 and those with certain health conditions such as chronic heart or lung disease. With those recommendations in mind and given the current situation, Cure HHT continues to refer to our HHT Center of Excellence physicians for their medical advisory as stated below.
Centers for Disease Control and Prevention (CDC):
- https://www.cdc.gov/coronavirus/2019-ncov/index.html (general and current COVID-19 information)
- How to Protect Yourself Against COVID-19: https://www.youtube.com/watch?time_continue=8&v=1APwq1df6Mw&feature=emb_title (video)
Update: March 5, 2020 | HHT and COVID-19: RISK FACTORS – LUNGS
Joint Statement – HHT Interventional Radiologists:
Justin McWilliams, MD – Co-Director HHT Center of Excellence UCLA
Scott Trerotola, MD – Director HHT Center of Excellence University of Pennsylvania
Miles Conrad, MD – Director, HHT Center of Excellence UCSF
Since this is a new virus, there is little published evidence about COVID-19. Overall, patients with HHT are not expected to be any more or less susceptible to the COVID-19 virus. The main concern with PAVMs is the loss of the filtering function of the lung, whereby bacteria and blood clots can pass into the arteries and cause well known complications. This is not a concern with a virus. Patients with untreated PAVMs large enough to cause low blood oxygen might be more at risk if infected with COVID-19 because their baseline lung function is not as good as those without PAVMs. This would not apply to those with small asymptomatic or treated PAVMs, for whom there is no reason to believe their ability to fight COVID-19 would be any different because their remaining lung is normal. Anyone with PAVMs requiring hospitalization for COVID-19 should have “bubble” filters on their IV, as recommended by the Cure HHT International Guidelines.
Most importantly, everyone with HHT should follow the CDC’s recommendations. These include:
- Avoid close contact with people who are sick
- Stay home when you are sick, except to get medical care
- Wash your hands often with soap and water for at least 20 seconds
- Cover your coughs and sneezes with a tissue
- Avoid non-essential travel to affected areas
Last, but not least, everyone with HHT should continue to follow the CDC’s recommendations regarding travel, hand washing, etc. and stay abreast of changes or additions to these as this situation unfolds.
For additional resources and information on COVID-19, please visit the CDC website. To review fact sheets and learn more about how you can help stop the spread of COVID-19, click here.